CPAC Equipment, Inc. (CPAC) is the world’s only developer and manufacturer of highvelocity hot air sterilizers (HVHA). On March 20, 2020, CPAC was requested to investigate whether their RH-series of HVHA sterilizers could be used to decontaminate previously used N95 respirators/masks. Published reports have demonstrated that dry heat processing is the preferred method for assuring both viral inactivation and N95 respirator performance efficacies are maintained for numerous re-use cycles. This tabletop unit can process approximately 100 masks per hour and can be located within a department, on a floor, or a wing of a healthcare facility where re-processing of N95 respirators is required. The unit requires only 110-115/15A service for operation.
Initial testing has demonstrated the RH-Pro11 can be modified (RH-N95) to achieve the time-temperature profile necessary for dry heat decontamination, meeting the documented conditions for coronavirus kill at 6 Log10 or greater while maintaining mask performance and function. Steps are currently being undertaken to procure the testing facilities necessary to demonstrate that these conditions do (1) inactivate the coronavirus (or a surrogate thereof) at a desired inactivation level and do (2) maintain mask structural integrity and performance efficacies at the required time-temperature threshold for 20 re-use cycles. Three stages have been identified for determining that the RH-N95 can provide the time and temperatures required for required viral kill and mask performance:
2. Conduct mask performance analysis on masks processed by the RH-N95 under prescribed mask decontamination cycle parameters to meet ASTM filtration and breathability performance standards required for new masks; and
3. Conduct viral and Mycobacterium kill efficacy studies to demonstrate that killing efficacy requirements are achieved at >6 Log10 reductions under prescribed decontamination cycle parameters.
Progress toward meeting these goals is presented below.
The Covid-19 pandemic has induced critical shortages of N95 masks which are used as the primary first line of defense to stop the virus from entering the respiratory system and manifesting disease. The world was not prepared for the magnitude of this crisis resulting in the use of inadequate masking or the attempt to decontaminate a previous used mask. For the latter to be successful in the decontamination process, the mask must be assured to have met the criteria for coronavirus destruction and for the maintenance (>95%) of the mask’s original performance standards. The FDA has just recently released its guidance document entitled “Enforcement Policy for Sterilizers, Disinfectant
Devices, and Air Purifiers During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency” (FDA1) which provides the guidance to provide the necessary safeguards of devices for mask re-processing. Meeting these FDA recommendations will allow devices not yet receiving the usually required FDA clearances to enter the market safely to relieve the N95 shortage. The CPAC RH-N95 meets those criteria allowing the unit to enter the market under this emergency authorization until our final quantitation testing is complete and FDA 510(k) issues marketing clearance.
Decontamination mechanisms for mask treatment include heat treatment, liquid disinfectants, UV, and chemical sterilants. Treatment mechanisms that introduce liquids to the mask run the high risk of severely lowering the mask’s filtration and breathability performance (Liao). This would include steam sterilization and any liquid disinfection method. Liquid disinfection/sterilization also introduces the problems of mask wetness, matting, residue retention, and possible mask deformation. Masks will need to be dried to eliminate pathogen pass-through via capillary action. Matting will impact on filtration and breathability. Residue retention could be deleterious to the wearer of the mask.
With all liquid disinfectants comes the inability to monitor the microbial efficacy performance of disinfectants since they are concentration and time dependent in their action. UV is not reliable due to its inability to penetrate the deep mask fibers and the “shadowing” interferences that preclude the UV from contacting pathogens.
Dry heat offers a simple mechanism that uses relatively low temperature (155°F) and moderate exposure times to decontaminate masks from coronavirus and other pathogens. Documented, peer-reviewed literature offers ample guidance in the temperature and exposure time required to inactivate coronavirus and other pathogens. No drying or residue removal is required of the process and at the relatively low temperatures required, little or no change in mask performance is expected.
Peer Reviewed Research
Data from peer-reviewed literature provides a strong indication of those dry heat temperatures and exposures required to inactivate coronavirus will maintain structural integrity and performance of the processed N95 respirator. This data has been instrumental in the construction of a decontamination cycle that should provide a > 6 Log10 reduction (>99.9999% reduction) in viral (Rabenau et al.) and bacterial concentrations (Sung and Collins). Although these microbial concentrations would seldom or ever be seen on a mask, these reduction levels provide the margin of safety necessary for mask re-use (FDA).
Extrapolation of the results presented in a report by the French Agency for Food and Occupational Health and Safety (ANSES) reveals that coronaviruses can be inactivated to a 6 Log10 reduction in 6 minutes at 63°C (145.5°F). This data is consistent with data provided by the World Health Organization for coronavirus (SARS) demonstrating an extrapolated 6 Log10 reduction in 22.5 minutes at 56°C (133°F). Rabenau et al. noted that heat treatment at 56°C for 30 minutes reduced a >7 Log10 SARS virus concentration to below the detection limit. Processing at 60°C for 30 min resulted in no infectious virus remaining (>7 Log10 reduction), regardless of the presence of any protein. The data gleaned from these references leads us to believe that dry heat temperatures between 65-70°C (149-158°F) are adequate to fully inactivate the coronavirus (>6 Log10 reduction) at an exposure of 30 minutes without damage to mask components or to the mask’s performance.
Additionally, a literature search revealed that Mycobacterium paratuberculosis, a Mycobacterium that is more thermally resistant than M. bovis, can be inactivated to a > 6 Log10 reduction in 6 minutes at 65°C (149°F) and at 68°F (155°C) in 2.2 minutes. Mycobacterium represents the most heat resistance of microbial species (other than spores and prions) and is used as the demonstrative surrogate for inactivation of other vegetative bacteria and of fungi, parasites, viruses and Mycobacterium. The demonstration of > 6 Log10 reduction of Mycobacterium would further assure user safety from the destruction other pathogens that might accompany a used mask.
The RH-Pro11 sterilizer has been designed to sterilize medical instruments at temperatures of 375°F for 6-minute (unwrapped) and 12-minute (wrapped) cycles. The device was originally granted 510(k) status from the U. S. Food and Drug Administration (FDA) in 1987 and 1988 as a Class II (Performance Standards) device. The RH-N95 consists of four trays each capable of holding 12 N95 masks (nested, double-stacked), allowing ninety-six N95 masks to be processed in little over an hour. The decontamination cycle time for N95 decontamination is presently set at 30 minutes at a temperature of 68°C (155°F) in accordance with the parameters gleaned from the literature cited above. Total processing is thirty-four minutes to allow mask heating before the decontamination cycle initiates. There is no water used in the process so the masks are dry at retrieval. The unit runs on 110-115V, 12 amps or 220-230V, 6 amps. It is a tabletop unit and requires no other utilities, allowing mask processing to occur department-by-department or floor-by-floor. The RH-Pro11 has been reconfigured to the RH-N95 to accommodate these lower, maskrequired temperatures of 155-156°F. Changes in software have enabled CPAC to create a specific cycle for mask decontamination. The graph in Figure 1 below is thermocouple temperature data (Labjack T7-Pro, T-Type thermocouples) derived from two N95 3M 8210 masks nested together under full load conditions, one thermocouple on the outside mask (Series 2) and the second on the inside mask (Series 1). Arrows indicate Process Start (left), Decontamination Cycle Initiation (middle), and Process Finish (right)
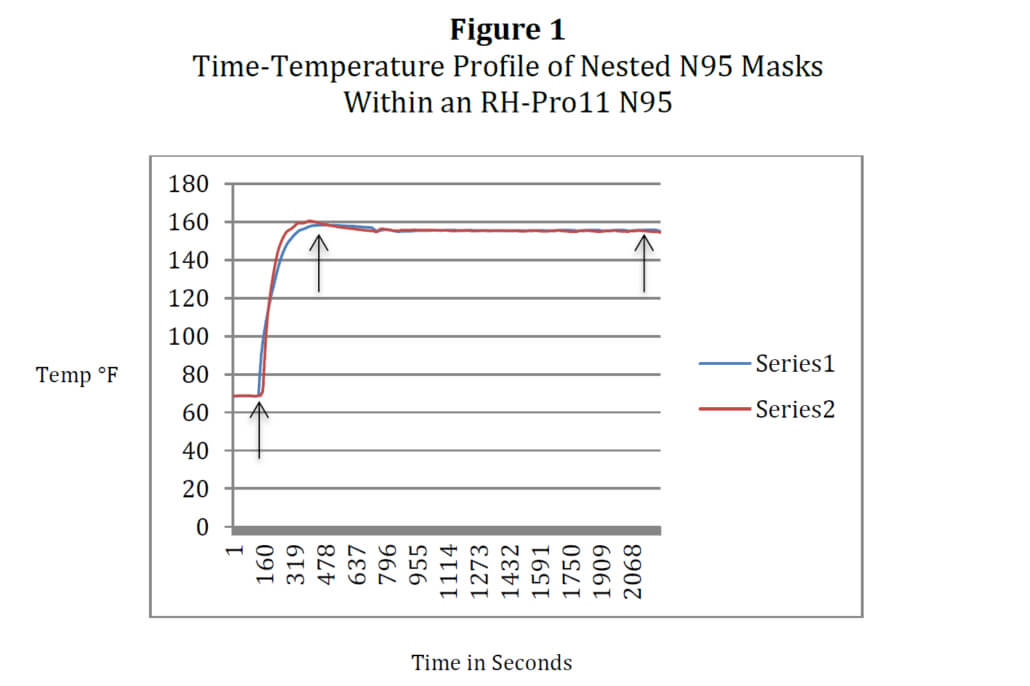
These conditions will allow the RH-N95 to process up to forty-eight masks per 30-minute cycle or close to one hundred masks per hour, enabling healthcare facilities to meet the demand for N95 masks placed on them by this epidemic. Two models of N95 masks have been tested for repetitive re-processing at this time: 3M N95 8210 and Kimberly-Clark Corporation PFR95). It should be further noted that by visual inspection, no heat incompatibilities were found through 20 cycles of re-processing in the elastic bands, foam nose fittings, and adhesives to detract from secure and form fit to nose and face.
To monitor conditions at the site of heat treatment, chemical indicators are typically used to determine if the conditions have been met to inactivate microorganisms. For the dry heat process these conditions are time and temperature. This is a new cycle specifically established for N95 masks. The temperatures are >200°F lower than the RH-Pro11 medical instrument sterilization cycles. No chemical indicator has been developed for this cycle and to do so would take research time and an FDA 510(k) clearance for marketing. A better and more direct approach will be taken using an autonomous temperature sensor that will document and record the time and temperature every 10 seconds at the location of mask placement. Data gleaned from this logger can be obtained real-time or stored and then downloaded upon completion of the cycle.
It should be noted that the time required for decontamination of the N95 masks in the RH-N95 may be significantly lower than the 30-minute exposure time derived from the literature. HVHA technology employed in the RH-Pro series of sterilizers has been demonstrated to reduce exposure times by 80% when compared to traditional static
(non-moving) air of dry heat sterilizers or dry heat ovens. Initial microbial inactivation studies by CPAC will use parameters found in the literature for its initial cycle settings. However, investigations will continue to determine if lower exposure times can provide a more expedited decontamination process.
Stage II: Mask Performance Analysis
Inherent for the re-use of an N95 mask is the requirement to assure that the decontamination process does not jeopardize the safety and health of the healthcare practitioner re-using the N95 mask. Specification performance of the mask’s integrity, composition, filtration and breathability are physical parameters that require analysis after the mask has been re-processed (FDA2). All visible indications from testing conducted by CPAC demonstrate the masks have not been altered by the decontamination process after fifteen 30-minute cycles, the limit of the cycles conducted to date. Our observations are consistent with a report from Stanford University which reported that after the N95 filtration fabric was dry heat treated at 75°C for 30 minutes after 20 cycles there was no change in filtration efficacy or pressure drop. Whole mask studies after 20 cycles showed no deformations in the mask or changes in elasticity of face/ear straps (Liao).
Further analytical testing remains to be conducted to assure standards for microbial filtration and breathability are met (FDA2). These studies will be initiated in the immediate future and it is fully expected that the masks will retain acceptable performance specifications to meet FDA 510(k) marketing clearance requirements.
Stage III: Microbiology Kill Efficacy Analysis
As with any decontamination process it is necessary to demonstrate quantitatively that the process is effective. Coronavirus inactivation reduction data reported the literature provides the assurance that the RH-N95 will be successful in viral inactivation at a 6 Log10 reduction or greater. Testing will be conducted using a coronavirus surrogate such as a Murine Hepatitis Virus to assure the coronavirus is inactivated by > 6 Log10 (FDA1,2). Additionally testing will be conducted to assure a reduction of >6 Log10 can be achieved on a Mycobacterium species to denote the destruction of other microbiological pathogens that may have also contaminated the mask during its use. These studies will be initiated shortly to meet FDA 510(k) marketing clearance requirements. Summary The RH-N95 has been allowed to begin marketing under the recent FDA Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers During the Coronavirus 2019 Public Health Emergency document (FDA1). Based on documented evidence that coronavirus can by inactivated at >6Log10 reduction at 155-156°F at an exposure of 30 minutes, an “N95” treatment cycle was created for RH-N95. These time-temperature parameters appear to cause no physical damage or inhibit mask filtration/breathability performance. Studies will be soon underway to quantify viral inactivation and mask performance in accordance with FDA 510(k) marketing clearance requirements.
References
Updated 3/4/2020
ANSES Opinion Request No 2020-SA-0037: March 9, 2020.
FDA¹, Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency
FDA², Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency: Guidance for Industry and Food and Drug Administration Staff, March 2020
Liao, L et al., Can N95 facial masks be used after disinfection? And for how many times? Report from the collaboration of Stanford University and 4C Air, Inc., March 25, 2020.
Rabenau, HF, et al., Stability and inactivation of SARS coronavirus. Med Microbiol Immunol 194: 1–6 (2005)
Sung, N and Collins, MT, Thermal Tolerance of Mycobacterium paratuberculosis. Applied And Environmental Microbiology, Mar: 999–1005, 1998.
World Health Organization. First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network.
Nelson S. Slavik, PhD
VP Research and Development, Senior Microbiologist
CPAC Equipment, Inc.
Leicester, NY